
Thrust 2: Plant-Derived Electronic Materials
We will use photosynthetic organisms as ‘factories’ to synthesize and pattern (semi)conducting polymers like polyaniline, polypyrrole, and polythiophene onto polymeric substrates. This approach will allow for the renewable synthesis of electronic materials, since light, air, and water are the only reagents needed for materials synthesis. Photosynthetic organisms produce oxidative byproducts as a product of respiration, and these can be used as chemical reagents for synthesizing traditional conducting polymers. Our objective is to produce organic electronic materials directly inside or adjacent to living matter; such integration will provide an interface to control function and/or sensor output. Thrust 2 will program cells to produce oxidative catalysts (e.g., peroxidases) and aromatic monomers (e.g., thiophene, pyrrole, aniline) followed by patterning of these cells into circuits within diverse polymeric substrates (films, fabrics, gels).
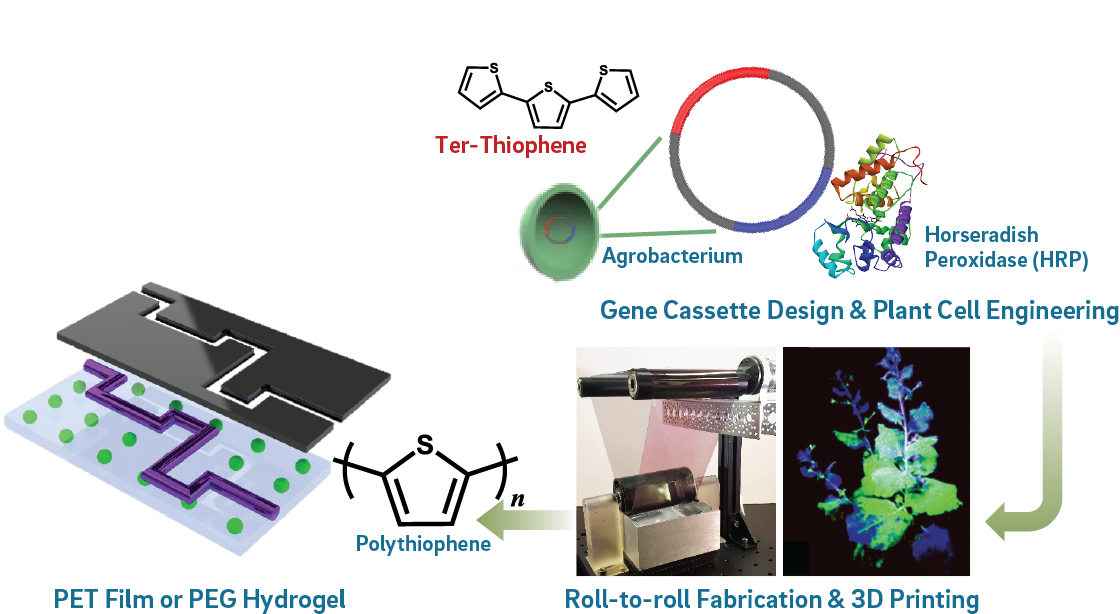
This thrust will focus on two elements: (1) Programming plant cells and microbes to act as polymer synthesis factories; and (2) Integration of such engineered cells into scalable materials manufacturing processes. Steinmetz, an expert in molecular farming and genetic modification of plants, will use genetic programming to catalyze the polymerization of conducting materials within plant cells and callus culture (plant stem cells). Feasibility of this concept has been established; reports demonstrate that conducting polymers can be grown in plants by feeding the monomers and initiators via the water source. Agroinfiltration and the use of plant viral expression systems has long been established in the pharmaceutical industry, and was used to produce the Ebola therapy, ZMAPP, used during the 2015 outbreak. As a new direction, we will use these concepts to produce cyborg organisms (i.e., living matter enhanced through synthetic parts). Steinmetz will use inducible optogenetic expression systems in plant cells to synthesize metalloenzymes like peroxidases or laccases; these enzymes will catalyze in planta synthesis of conducting polymers like polyaniline or polypyrrole. Pokorski has demonstrated enzymatic polymer synthesis of polyaniline and its associated patterning through additive manufacturing. Concurrently, Burkart and Mayfield will design expression cassettes for plant cells that will synthesize pyrrole, thiophene, and aniline derivatives. Genes for monomers and catalysts will be integrated into the plant genome using plant cell agroinfiltration, otherwise plant viral gene delivery will lead to transient expression. These genes will be turned ON using optogenetic methods based on phytochrome B and one of its interacting factors (PIF6). In the presence of red light (660 nm), high yields of the monomers and catalysts will be achieved. These genes can then be turned off under far-red light (740 nm). We will introduce stimuli- induced, self-replicating elements to produce self-renewing or -healing living matter. Callus cultures will include stomatal guard structures (which open and close upon external stimuli like vacuum or salt) thus enabling a gate between internally synthesized conducting polymers. Cell pellets will be characterized using a battery of techniques including protein assays to verify enzyme expression, as well as NMR, mass spectrometry, spectroscopy, and 4-point electrical probe testing and XPS analysis to study the polymers versus commercially available synthetic material. In addition, we will gain insights into the dynamics of the synthesis process by making use of fluorescent monomers and longitudinal imaging (macroscopic and microscopic).
J. Golden and S. Golden will complement these efforts by utilizing cyanobacteria in directed evolution experiments to improve the oxidation potential of new peroxidases (unpublished, but first in class in cyanobacteria). As new catalysts are synthesized, Burkart and Mayfield will develop methods to genetically synthesize new biosynthetic monomers (as guided by Lipomi); pyrroles and thiophenes will be scaffolds since they have known biosynthetic pathways. This will allow the team to design catalyst/monomer pairs to polymerize next generation (semi)conducting polymers using living materials.
Lipomi’s expertise will be vital to achieving integration of engineered plant cells with polymeric substrates. An expert in the design of π-conjugated (semiconducting and conducting) polymers with properties inspired by biological tissue (stretchability, biodegradability, and self-repair), Lipomi has developed scalable roll-to-roll coating systems for thin films and fabrics. Lipomi will use this process to deposit engineered plant cells onto transparent poly(ethylene terephthalate) (PET) films. These films will then be patterned by red-light photolithography to activate biosynthesis of conducting polymers. We anticipate using a variety of substrates like stretchable fabrics and elastomeric gel membranes in addition to polymer films. The ability to integrate living components into solid or hydrated films of such materials offers several exciting new avenues of stimulus response. For example, the living components could affect the direct synthesis/repair of these materials by oxidative polymerization of various aromatic heterocycles. They could also serve as the source of stimuli-responsive modulation of the conductivity (i.e., gating by light, strain, and the presence of ions, proteins, and metabolites) by changes in pH, and excretion of ions, metabolites, and proteins. This capability could have a transformative impact on organic bioelectronic sensors and other devices, particularly in wearable and implantable form factors. We anticipate that these materials will find use in wearable sensors and on-demand synthesis of electronic components in resource limited environments.
